Arxius de Miscel·lània Zoològica. Volum 23 (2025) Pàgines: 77-85
Dietary habits of the wrinkle-lipped free-tailed bat Mops plicatus in Cambodia
Sin, S., Meas, S., Khin, C., Uk, O. N., Thi, S.
DOI: https://doi.org/10.32800/amz.2025.23.0077Descarregar
PDFCita
Sin, S., Meas, S., Khin, C., Uk, O. N., Thi, S., 2025. Dietary habits of the wrinkle-lipped free-tailed bat Mops plicatus in Cambodia. Arxius de Miscel·lània Zoològica, 23: 77-85, DOI: https://doi.org/10.32800/amz.2025.23.0077-
Data de recepció:
- 08/01/2025
-
Data d'acceptació:
- 15/04/2025
-
Data de publicació:
- 08/05/2025
-
Compartir
-


-
Visites
- 496
-
Descàrregues
- 156
Abstract
Dietary habits of the wrinkle-lipped free-tailed bat Mops plicatus in CambodiaThe role of bats in regulating pest insect populations in agricultural ecosystems has recently emerged as a topic of global interest to farmers and conservationists. However, the diet of bats in Cambodia remains largely unexplored. We analysed faecal pellets of Mops plicatus to understand the role of this bat species in suppressing agricultural pests. Faecal pellets were collected, during the late wet and early dry seasons, from La Ang Reach Trop and Phnom Preah Kuhear Luong in Battambang and Kampot Provinces, respectively. Seven insect orders and Acari were identified in the diet of M. plicatus individuals. This bat species mainly consumed Hemiptera (Delphacidae) with 76.78 ± 30.35 % and Coleoptera with 13.02 ± 22.09 % of its diet across all regions and seasons. The less frequent orders were Hemiptera (Cicadellidae), Lepidoptera, Diptera, Hymenoptera, Odonata, Siphonaptera, and Acari. We found a significant difference in the diet of M. plicatus between seasons, and between both areas. The results suggest that M. plicatus plays a significant role in the predation of Delphacidae, contributing to the pest regulation in rice paddy fields and increasing rice production. Prioritising the conservation and management of M. plicatus and its habitats is critical for maintaining its ecosystem services and economic value.
Key words: Pest insects, Paddy field, Bat conservation, Delphacidae, Cave bat, Limestone
Resumen
Hábitos alimentarios del murciélago caerepon de nariz arrugada Mops plicatus en CamboyaEl papel de los murciélagos en la regulación de las poblaciones de insectos plaga en los ecosistemas agrícolas se ha convertido recientemente en un tema de interés mundial para agricultores y conservacionistas. Sin embargo, la dieta de los murciélagos de Camboya permanece en gran medida inexplorada. Analizamos las egagrópilas fecales de Mops plicatus para comprender el papel de esta especie de murciélago en la supresión de plagas agrícolas. Se recogieron egagrópilas fecales, a finales de la estación húmeda y a principios de la seca, en La Ang Reach Trop y Phnom Preah Kuhear Luong, en las provincias de Battambang y Kampot, respectivamente. Se identificaron siete órdenes de insectos y Acari en la dieta de los individuos de M. plicatus. Esta especie de murciélago consumió principalmente Hemiptera (Delphacidae) con un 76,78 ± 30,35 % y Coleoptera con un 13,02 ± 22,09 % de su dieta en todas las regiones y estaciones. Los órdenes menos frecuentes fueron Hemiptera (Cicadellidae), Lepidoptera, Diptera, Hymenoptera, Odonata, Siphonaptera y Acari. Encontramos una diferencia significativa en la dieta de M. plicatus entre estaciones, y entre ambas zonas. Los resultados sugieren que M. plicatus desempeña un papel significativo en la depredación de Delphacidae, contribuyendo a la regulación de plagas en los arrozales y aumentando la producción de arroz. Dar prioridad a la conservación y gestión de M. plicatus y sus hábitats es fundamental para mantener sus servicios ecosistémicos y su valor económico.
Palabras clave: Insectos plaga, Arrozales, Conservación de murciélagos, Delphacidae, Murciélago cavernícola, Piedra caliza
Resum
Hàbits alimentaris del ratpenat cuallarg de nas arrugat Mops plicatus a CambodjaEl paper dels ratpenats en la regulació de les poblacions d'insectes plagues als ecosistemes agrícoles ha sorgit recentment com un tema d'interès global per als agricultors i els conservacionistes. No obstant això, la dieta dels ratpenats a Cambodja segueix sent en gran part inexplorada. Hem analitzat els pellets fecals de Mops plicatus per entendre el paper d'aquesta espècie de ratpenat en la supressió de plagues agrícoles. Es van recollir pellets fecals, durant les estacions humides tardanes i primerenques, de La Ang Reach Trop i Phnom Preah Kuhear Luong a les províncies de Battambang i Kampot, respectivament. Es van identificar set ordres d'insectes i Acari a la dieta dels individus de M. plicatus. Aquesta espècie de ratpenat consumia principalment hemiptera (Delphacidae) amb un 76,78 ± 30,35 % i coleòpters amb un 13,02 ± 22,09 % de la seva dieta a totes les regions i estacions. Els ordres menys freqüents eren Hemiptera (Cicadellidae), Lepidoptera, Diptera, Hymenoptera, Odonata, Siphonaptera i Acari. Hem trobat una diferència significativa en la dieta de M. plicatus entre estacions i entre ambdues zones. Els resultats suggereixen que M. plicatus té un paper important en la depredació de Delphacidae, contribuint a la regulació de plagues als arrossars i augmentant la producció d'arròs. Prioritzar la conservació i la gestió de M. plicatus i els seus hàbitats és fonamental per mantenir els seus serveis ecosistèmics i el seu valor econòmic.
Paraules clau: Insectes plaga, Arrossar, Conservació de ratpenats, Delfàcids, Ratpenat de les coves, Pedra calcària
Introduction
Bats are the second-most diverse group of mammals globally, with over 1,450 species worldwide and 81 species of which were recorded in Cambodia (Csorba and Furey 2022, Simmons and Cirranello 2023). In recent years, bats have gained attention from both farmers and conservationists due to their critical role in regulating insect pest populations in agricultural systems. Insectivorous bats are capable of consuming insects up to 80-100 % of their body weight each night (Kunz and Stern 1995, Kunz et al 2011), highlighting their significant ecological contribution. As natural pest control agents, bats provide essential ecosystem services, which have prompted researchers to emphasize their importance in sustainable agricultural practices (Kunz et al 2011). However, habitat fragmentation and land clearing, driven by broadacre cropping, urbanisation, limestone quarrying for cement and construction materials, and mining pose substantial threats to many bat species in Cambodia (Furey et al 2016). These human activities often lead to the destruction of critical roosting and foraging habitats, further endangering bat populations and compromising the ecosystem services they provide. This situation is due to a lack of positive public perceptions and understanding of the ecosystem services and value of bats to the agricultural sector (Williams-Guillén et al 2015, Hoffmaster et al 2016).
Analysis of bat diets offers insights that can highlight the valuable pest control services they provide in agricultural systems, which can contribute to changing public perceptions and promote the conservation of their habitats (Lima and Bastos 2021). However, a lack of knowledge regarding the diets of many Cambodian insectivorous bats has rendered it challenging to establish a link between their potential pest consumption service and farm profits, thereby impeding the conservation of bats and their habitats in agroecosystems.
The wrinkle-lipped free-tailed bat Mops plicatus is a widespread insectivorous bat species found from eastern India to Sri Lanka, southern China and throughout Southeast Asia (Heaney 1998, Molur et al 2002, Simmons 2005). This bat species is particularly well-known for its habitation of caves, where it forms large colonies of up to several million individuals (Hillman 1999). The evening emergence of these significant colonies is of interest to millions of tourists who visit annually at Sampeu Hill in Battambang Province, Cambodia (Furey and Racey 2016). Mops plicatus is capable of flying up to 60 km from their roosting sites, and foraging over an area of 40 to 1,740 km2 each night (Srilopan et al 2025). This foraging range could prove to be a valuable contribution to the control of pest insects in large agricultural landscapes (McFarlane et al 2015). In Thailand, this bat species plays a crucial role in control of white-backed and brown planthoppers (Leelapaibul et al 2005, Srilopan et al 2018). These planthoppers are well-known for their destructive impact on rice crops throughout Asia (Catindig et al 2009). A total of 1.3 million individuals of M. plicatus have been estimated to feed on approximately ten million planthoppers (Srilopan et al 2018), with an average daily intake of up to 11.7 tons of insects. The ecosystem service provided by this bat species helps to protect rice yield losses of up to USD 1.2 million, which could provide meals for more than 26,000 people each year (Wanger et al 2014).
A total of 12 colonies of M. plicatus have been recorded in Cambodia, seven of which are located in limestone caves. These seven colonies represent an extensive range in population size, with recorded colonies ranging from 0.37 to 1.8 million bats in northwestern (Battambang Province) and southern (Kampot Province) Cambodia (Furey et al 2016). This represents approximately 97 % of the national colony, which is estimated to be 6.37 million individuals (Yim and Mackie 2009). Despite this, dietary analyses of M. plicatus has yet to be conducted in Cambodia. This study, therefore, seeks to address these gaps through seasonal dietary analysis of two large colonies of M. plicatus to understand their role in agricultural pest insect management. Furthermore, the study aims to promote the long-term conservation of this species by providing reliable scientific data for decision-makers and by raising awareness of the pest suppression benefits of this bat species.
Materials and methods
Study area
Faecal samples were collected during the late wet (September to October 2023) and the early dry seasons (January to February 2024). The study focused on two large colonies of M. plicatus; La Ang Reach Trop and Phnom Preah Kuhear Luong (fig. 1).

Fig. 1. Locations of the two study caves of Mops plicatus: La Ang Reach Trop, Battambang Province, in northwestern and Phnom Preah Kuhear Luong, Kampot Province, southern Cambodia.
Fig. 1. Ubicación de las dos cuevas de estudio de Mops plicatus: La Ang Reach Trop, provincia de Battambang, en el noroeste; y Phnom Preah Kuhear Luong, provincia de Kampot, sur de Camboya.
The first colony is located in La Ang Reach Trop (12.955132 ºN, 103.135461 ºE) Banan district, Battambang Province, in northwestern Cambodia. It is estimated that approximately 900,000 individuals of M. plicatus inhabit this cave (Furey et al 2016). The landscape surrounding the cave is predominantly characterized by agricultural activities, including rice paddy fields, maize cultivation, longan orchards, and other local crops. The annual rainfall in Battambang is 1,843 mm, with an average temperature of 33 ºC (1951-2018). The warmest month is April, with an average temperature of 36 ºC. The wet season begins in May and lasts until October, with a maximum rainfall of 220-302 mm. The period of lowest precipitation occurs between November and April, which is referred to as the dry season (Furey et al 2016).
The second colony is situated in Phnom Preah Kuhear Luong (10.663283 ºN, 104.537641 ºE) Tuk Meas district, Kampot Province southwestern Cambodia. It is estimated that approximately 370,000 individuals of M. plicatus inhabit the cave of Phnom Preah Kuhear Luong, which is conserved by the local community for guano harvesting (Furey et al 2016). The natural landscape surrounding the cave is characterised by karst mountains, limestone cliff vegetation, rice paddy fields, cucumber farms and houses. The climate of Kampot is tropical, with an average annual rainfall of 2,000 mm and a temperature of 32 ºC. May to October refers to the wet season with rainfall ranging from 200 to 335 mm. The dry season begins in November to April with the lowest rainfall (ADB 2021). The distance between the two colonies is approximately 310 km.
Bat faecal sampling and analysis
Faecal pellets were collected using four pieces of one-meter squares of white plastic, which were positioned on the ground beneath bat roosts overnight (18:00 to 6:00h). Sampling was conducted over 32 nights at each cave. The faecal pellets that had been deposited on each piece of plastic were collected using soft forceps and preserved in labelled e-tubes containing 70 % ethanol. These samples were then transported to the laboratory for analysis. Each piece of white plastic was cleaned thoroughly to ensure that no residual material remained for reuse purposes.
The faecal pellets were dissected at the Zoological Museum of the Centre for Biodiversity Conservation, Royal University of Phnom Penh. Each pellet was individually placed on a Petri dish for observing the prey fragments. A few drops of glycerol were applied to the pellet using a plastic pipette to prevent moisture loss, facilitate the dissection process, and minimize damaging the prey fragments during observation (Sin et al 2020). All prey fragments were examined under a stereo microscope (Olympus SZ51) and identified to order and family levels using key references (Whitaker 1988, McAney et al 1991, Wilson and Claridge 1991, Johnson and Borror 2005, Pokhrel and Budha 2014, Waghiiwimbom et al 2019, Sin et al 2020). In this study, Hemipterans were identified into the families Delphacidae (planthoppers) and Cicadellidae (leafhoppers) based on distinct morphological characteristic. Delphacids were identified by their movable spur on the hind tibia and reduce wing venation (fig. 2C, 2D) (Bartlett et al 2011). Cicadellids, on the other hand, were distinguished by their rows of spines on the hind tibia (fig. 2F) (Dietrich 2005).

Fig. 2. Mops plicatus and its food sources: A, lateral morphology of Mops plicatus (© S. Hun); B, D, fragment of Delphacidae (planthopper); B, head; C, wing; D, hind-leg; E, wing of Hemiptera (true bug); F, legs of Cicadellidae (leafhopper); G, wing of Coleoptera (beetle); H, wing scales of Lepidoptera (moth); I, head of Diptera (mosquito); J, wing of Hymenoptera (wasp); K, wing of Odonata (damselfly); L, Siphonaptera (flea); M, Acari (mite).
Fig. 2. Mops plicatus y sus fuentes de alimento: A, morfología lateral de Mops plicatus (© S. Hun); B, D, fragmento de Delphacidae (saltaplantas); B, cabeza; C, ala; D, pata trasera; E, ala de Hemiptera (chinche verdadera); F, patas de Cicadellidae (saltahojas); G, ala de Coleoptera (escarabajo); H, escamas del ala de Lepidoptera (polilla); I, cabeza de Diptera (mosquito); J, ala de Hymenoptera (avispa); K, ala de Odonata (libélula); L, Siphonaptera (pulga); M, Acari (ácaro).
Data analysis
The presence or absence of identified prey orders and families consumed by M. plicatus was recorded for each faecal pellet analysed (Leelapaibul et al 2005). The percentage frequency and percentage volume of each prey item were estimated following the methodology outlined by Whitaker (1988). To compare the food sources in the diet across the two study caves and between dry and wet seasons, a PERMANOVA was performed. Additionally, the SIMPER method was used to identify significant differences in food sources across seasonal and spatial variations. The normality of the data was assessed using the Shapiro-Wilk test. All statistical analyses were conducted in R Studio 4.4.1 for Windows, with a significance level set at p < 0.05.
Results
Dietary analysis
A total of 320 faecal pellets were analysed to identify the food sources of M. plicatus, with 160 pellets collected from each province during both the wet and dry seasons. The identified food sources were categorized into seven insect orders: Hemiptera (Delphacidae and Cicadellidae), Coleoptera, Lepidoptera, Diptera, Hymenoptera, Odonata, and Siphonaptera, as well as Acari (fig. 2).
The study revealed that, based on the percent volume combined across all locations and seasons, M. plicatus primarily fed on Delphacidae, which accounted for an average consumption of 76.78 ± 30.35 %, making it the most important food source. This was followed by Coleoptera, which constituted for 13.02 ± 22.09 % of the diet. The least significant food sources were Hemiptera (Cicadellidae), Lepidoptera, Diptera, Hymenoptera, Odonata, Siphonaptera, and Acari (table 1).

Table 1. Percentage volume (mean ± SE) of all food sources consumed by Mops plicatus during the wet and dry seasons at La Ang Reach Trop in Battambang Province and Phnom Preah Kuhear Luong in Kampot Province, Cambodia.
Tabla 1. Porcentaje de volumen (media ± EE) de todas las fuentes de alimentos consumidas por Mops plicatus durante las estaciones húmedas y secas en La Ang Reach Trop en la provincia de Battambang y en Phnom Preah Kuhear Luong en la provincia de Kampot, Camboya.
In the analysis by percent frequency, three food sources were found to contribute significantly to the diet of M. plicatus: Delphacidae (95 ± 21.8 %), Coleoptera (68 ± 46.5 %), and Lepidoptera (44 ± 49.7 %). The following food sources were less significant: Hemiptera (18 ± 38.1 %) and Hymenoptera (8 ± 26.9 %). The least significant food sources were Diptera, Cicadellidae, Odonata, Siphonaptera, and Acari (table 2).
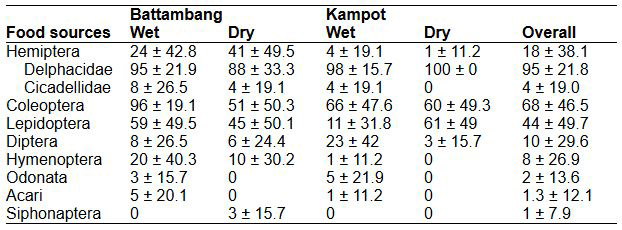
Table 2. Percentage frequency (mean ± SE) of all food sources consumed by Mops plicatus during the wet and dry seasons at La Ang Reach Trop in Battambang Province and Phnom Preah Kuhear Luong in Kampot Province, Cambodia.
Tabla 2. Frecuencia porcentual (media ± EE) de todas las fuentes de alimentos consumidas por Mops plicatus durante las estaciones húmedas y secas en La Ang Reach Trop en la provincia de Battambang y en Phnom Preah Kuhear Luong en la provincia de Kampot, Camboya.
Seasonal and spatial variation in diet
The analysis of seasonal dietary composition showed that Delphacidae, Coleoptera, and Lepidoptera were the most important food sources for M. plicatus, based on both percentage volume and percentage frequency. Delphacidae and Hemiptera showed significantly higher consumption during the dry season than during the wet season in Battambang (all p-values < 0.001). In contrast, the dietary intake of Coleoptera increased significantly in the diet during the wet season, replacing the reduced consumption of Delphacidae and Hemiptera (p-value < 0.001). In Kampot, significant differences were observed in the proportions of Lepidoptera and Diptera between the dry and wet seasons (all p-values < 0.001).
The PERMANOVA analysis revealed significant differences in the food sources of M. plicatus between the dry and wet seasons (R² = 0.017, p-value = 0.003), as well as between Battambang and Kampot (R² = 0.077, p-value = 0.001) (fig. 3). Specifically, six food sources showed significant seasonal variation, including Coleoptera, Hemiptera, Delphacidae, Diptera, Lepidoptera, and Odonata (all p-values < 0.05). Furthermore, a comparison between the two study areas revealed significant differences in five food sources: Delphacidae, Coleoptera, Hemiptera, Hymenoptera, and Diptera (all p-values < 0.05).

Fig. 3. Two-dimensional MDS ordination of the diet of Mops plicatus in the seasonal and spatial variation, La Ang Reach Trop in Battambang Province (BTB) and Phnom Preah Kuhear Luong in Kampot Province (KP), Cambodia.
Fig. 3. Ordenación MDS bidimensional de la dieta de Mops plicatus en la variación estacional y espacial, La Ang Reach Trop en la provincia de Battambang (BTB) y Phnom Preah Kuhear Luong en la provincia de Kampot (KP), Camboya.
Discussion
Bat diet
This study provides the first insights into the diet of M. plicatus in Cambodia. Our findings indicate that the primary food sources consumed by M. plicatus were Delphacidae, followed by Coleoptera and Lepidoptera, while the least important food sources were Hemiptera, Hymenoptera, Diptera, Cicadellidae, Odonata, Siphonaptera, and Acari. These results align with previous studies conducted in Thailand, where M. plicatus was primarily observed to feed on Homoptera (Delphacidae), along with Coleoptera, Lepidoptera, Hemiptera, and Diptera (Leelapaibul et al 2005, Srilopan et al 2018). Srilopan et al (2018) suggested that M. plicatus colonies play a crucial role in regulating the insect pest Nilaparvata lugens (Delphacidae) in Thailand. Nilaparvata lugens has been widely documented in Cambodia's lowland regions, particularly in areas with extensive rice plantations (Matsukawa et al 2018). This insect species is a major pest of rice (Oryza sativa) in Southeast Asia, contributing to significant yield losses and economic impacts. It feeds on rice phloem sap, which leads to nutrient depletion and honeydew-induced sooty mold growth. N. lugens also as a vector for rice viruses, such as rice grassy stunt virus and rice ragged stunt virus, compounding yield losses ranging from 10% to 60 % (Bottrell and Schoenly 2012, Hu et al 2016). In this study, Delphacidae was the most common food source, present in both the dry and wet seasons. This is likely due to the presence of active rice plantations in the study areas, which provide an abundant source of Delphacidae.
Seasonal and spatial variation in diet
The investigation revealed significant seasonal variation in the diet of Delphacidae, Coleoptera, and Hemiptera in Battambang, with a marked increase in the percentage volume consumed during the dry season. In contrast, no significant differences in diet volume were observed for these three food sources in Kampot. However, the proportion of Delphacidae consumed during the dry season was notably higher than that observed during the wet season in Kampot. The observed pattern of higher Delphacidae consumption during dry season in both areas, despite fallow rice paddy fields, may be linked to the seasonal dynamics of rice cultivation and the ecological interactions within agroecosystems. During the dry season, rice fields are typically fallow, and Delphacidae may concentrate in remnant habitats such as weeds, grasses, or irrigated fields, making them more accessible to natural enemies (Lu et al 2015). Additionally, the scarcity of alternative prey during this period may force predators to focus more intensively on Delphacidae, leading to a higher proportion of consumption (Settle et al 1996). In contrast, during the wet season, when rice paddy fields are actively cultivated, Delphacidae populations thrive due to the availability of suitable host plants, but the proportion consumed by natural enemies may be lower due to the presence of alternative prey and disruptions caused by heavy rainfall (Heong et al 1992, Bottrell and Schoenly 2012). These studies aligned with our finding, the decrease in Delphacidae consumption during the wet season may be attributed to the increased availability of other food sources. Additionally, M. plicatus exhibited a greater diversity of food sources during the wet season (9-10 food sources) compared to the dry season (5-7 food sources). A typically seasonal ecosystem is characterized by a rainy period, during which food resources are abundant and varied, and a dry season, when food availability is limited (Tonkin et al 2017, Varpe 2017). Consequently, seasonal fluctuations in food resources are expected to influence the dietary patterns of individual bat species.
The food consumption of M. plicatus was found to be similar between Battambang and Kampot, with arthropod groups making up the majority of their diet. However, a comparison of the two regions revealed significant differences in the proportion of five food sources, including Delphacidae, Lepidoptera, Hemiptera, and Hymenoptera. These findings suggest that bats may adjust their dietary preferences based on the variety and availability of prey in their foraging habitat (Srinivasulu and Srinivasulu 2005). Battambang and Kampot are located at considerable distances from each other, which could account for the observed differences, likely due to the distinct ecological features and climatic conditions of each region. The ecological framework plays a crucial in influencing the diversity and availability of prey for M. plicatus. Variation in prey preferences were also observed between the different seasons and regions. This supports the idea that Battambang and Kampot, being geographically and ecologically distinct, provide varying prey resources. Our findings align with previous research showing that the diet composition of M. plicatus can vary significantly across different habitats and times (Kurta and Whitaker 1998).
Conservation implications
The findings of this study highlight the ecological importance of M. plicatus in Cambodia as a natural regulator of insect populations, particularly Delphacidae, which includes the economically significant rice pest N. lugens. By preying on N. lugens, M. plicatus provides an essential ecosystem service that supports rice production and reduces the need for chemical pesticides, thereby promoting sustainable agricultural practices (Bottrell and Schoenly 2012, Srilopan et al 2018). The observed dietary flexibility of M. plicatus highlights its ecological importance in maintaining insect population balance, particularly in regions with extensive rice cultivation. However, this reliance on agricultural landscapes also makes the species vulnerable to habitat degradation, pesticide use, and climate change, which can disrupt prey availability and foraging success. To ensure the long-term survival of M. plicatus, conservation efforts should focus on preserving foraging habitats, particularly in rice-growing regions, and protecting roosting sites such as caves and abandoned structures. Additionally, integrated pest management strategies that minimize pesticide use and promote natural pest control by bats should be encouraged.
The findings of this study can be incorporated into awareness and education programs to highlight the ecological and economic benefits of M. plicatus. For example, agricultural extension services in Cambodia could educate farmers about the role of bats in controlling rice pests and the importance of reducing pesticide use to support bat populations. Public awareness campaigns and integrated pest management strategies that emphasize the role of bats in pest control can further enhance conservation outcomes. Long-term monitoring of M. plicatus populations and their dietary patterns is essential to assess the impacts of environmental changes and inform adaptive management strategies. By safeguarding M. plicatus, we not only protect a keystone species but also enhance ecosystem services that benefit both biodiversity and human livelihoods. This study underscores the need for interdisciplinary collaboration between ecologists, conservationists, and agricultural stakeholders to develop holistic approaches that balance agricultural productivity with biodiversity conservation.
Conclusion
The results of our study indicate that Delphacidae is a primary food source for M. plicatus in both Battambang and Kampot Provinces, Cambodia. The colonies of M. plicatus play a significant role in regulating insect pest populations, particularly Delphacidae, in rice paddy fields throughout the year. Consequently, it can be postulated that increased rice production may be associated with the presence of M. plicatus colonies. Given the population decline of M. plicatus caused by illegal hunting and limestone quarrying, it is, therefore, crucial to prioritize the conservation and management of this species and its habitats in order to sustain its ecosystem services and economic value.



















